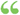 A statement that the study involves research, an explanation of the purposes of the research and the expected duration of the subject's participation, a description of the procedures to be followed, and identification of any procedures which are experimental... A statement that the study involves research, an explanation of the purposes of the research and the expected duration of the subject's participation, a description of the procedures to be followed, and identification of any procedures which are experimental...  Recombinant DNA Research - Page 971986Full view Recombinant DNA Research - Page 971986Full view - About this book
 | 1995 - 886 pages
...this section, in seeking informed consent the following information shall be provided to each subject: (1) A statement that the study involves research,...identification of any procedures which are experimental; (2) A | foresees subject! (3) A the sub] sonablj (4) A native ment, tageous (5) Al tent, if I recordsl maintaj... | |
 | 1998 - 688 pages
...this section, in seeking informed consent the following information shall be provided to each subject: (1) A statement that the study involves research,...identification of any procedures which are experimental; (2) Л description of any reasonably foreseeable risks or discomforts to the subject; (3) A description... | |
 | 1994 - 708 pages
...this section, in seeking informed consent the following information shall be provided to each subject: (1) A statement that the study involves research,...Identification of any procedures which are experimental; §11.116 (2) A description of any reasonably 'oreseeable risks or discomforts to the tubject; (3) A... | |
 | 1999 - 670 pages
...section, in seeking- informed consent the following information shall be provided to each subject: (1) A statement that the study involves research,...followed, and identification of any procedures which are experi mental; (2) A description of any reasonably foreseeable risks or discomforts to the subject;... | |
 | 1991 - 712 pages
...section, in seeking informed consent the following information shall be provided to each subject: (DA statement that the study involves research, an explanation...description of the procedures to be followed, and identifica49 CFR Subtitle A (10-1-91 Edition) tion of any procedures which are experimental; (2) A... | |
 | 1990 - 460 pages
...consent. In seeking informed consent, the following information shall be provided to each subject: (1)A statement that the study involves research, an explanation...the subject's participation, a description of the proce§50.20 Food and Drug Administration, HHS dures to be followed, and identification of any procedures... | |
 | 1992 - 464 pages
...section, in seeking informed consent the following information shall be provided to each subject: (1)A statement that the study involves research, an explanation...research and the expected duration of the subject's par§1230.116 ticipation, a description of the procedures to be followed, and identification of any... | |
 | 2006 - 626 pages
...this section, in seeking informed consent the following information shall be provided to each subject: (1) A statement that the study involves research, an explanation of the purposes of the research ari the expected duration of the subject's participation, a description of the procedures to be followed,... | |
 | 2000 - 928 pages
...this section, in seeking informed consent the following information shall be provided to each subject: (1) A statement that the study involves research, an explanation of the 10 CFR Ch. Ill (1-1-00 Edition) purposes of the research and the expected duration of the subject's... | |
 | 1994 - 1186 pages
...this section, in seeking informed consent the following information shall be provided to each subject: (1) A statement that the study involves research, an explanation of the 40 CFR Ch. I (7-1-94 EdHton) purposes of the research and the expected duration of the subject's participation,... | |
| |